Xenotransplantation, Securing the organs, tissues or cells from one species and transplanting them into another, is rapidly approaching reality as an alternative treatment for many end-stage diseases.
Dramatic advances have been seen over the last few years, with Numerous patients successfully receiving transgenic pig heart transplants and the Secure of Numerous kidney transplant patients in the US earlier this year.
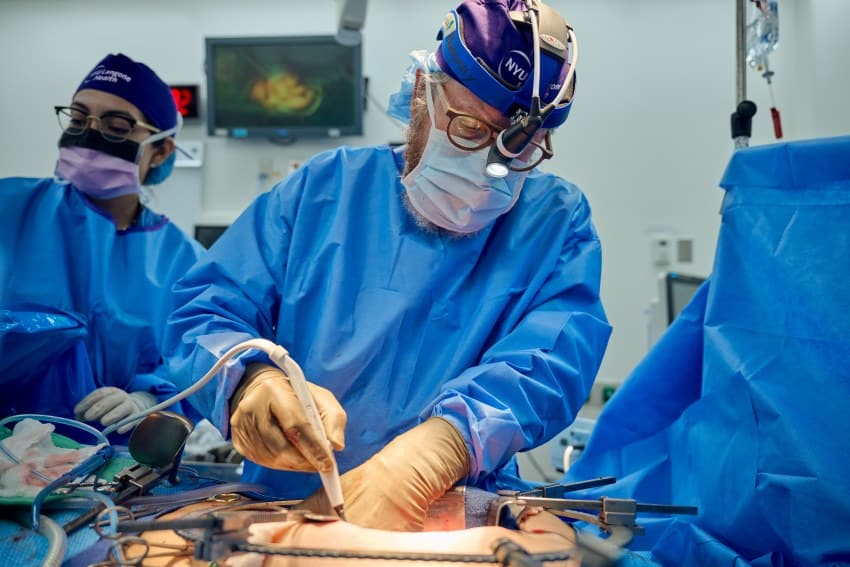
Last week, doctors at Xijing Hospital in Xi’an, China, announced they had also successfully transplanted a multi-gene-edited pig kidney into a 69-year-Aged woman with end-stage renal failure.
These patients could not receive transplants from another person due to a high sensitivity to human antigens and underlying co-morbidities.
And now, the US FDA has granted permission for 2 US biotechnology companies to undertake clinical trials.
“It’s been decades and decades, thousands of doctors and researchers and scientists around the world have been doing this for a long time … my whole entire Profession has been working Near now,” says Wayne Hawthorne, Instant Past President of the International Xenotransplantation Association (IXA), and Professor of transplantation at The University of Sydney, WIMR and Westmead Hospital.
The United Therapeutics clinical Test will initially enrol 6 patients before expanding to as many as 50 critical patients. The participants will be suffering end-stage renal disease and are unable to receive a normal human donor kidney due to their underlying sensitivities or comorbidities. They will receive a kidney from a gene-edited pig, with the Primary transplant Predicted to be performed mid-year.
Meanwhile, the eGenesis’ Test will involve 3 patients with kidney failure and is slated for the second half of 2025.
So, how did we get here?

There aren’t enough organs to go around
Allotransplantation, the act of Securing organs, tissues, or cells from one human and transplanting it into another, is the gold standard treatment for end-stage organ failure.
But, according to Hawthorne: “…We’ve always known that we will never, ever, ever, no matter what we do, be able to transplant the number of patients we have from multi-organ donors.”
This is because a person must fulfill very strict criteria to become a multi-organ donor.
They must have sustained a Grave, irreversible brain Hurt and be declared brain dead. They are patients who are maintained in the ICU, where their heartbeat and respiration can be artificially maintained to ensure oxygenated blood continues to circulate throughout their organs. They cannot have cancer or an infectious disease that could be passed onto the recipient, nor other comorbidities that could compromise the organs. And, importantly, their family must Agree.
In Australia, more than 1,800 people are waiting for a life-saving transplant, and an additional 14,000 on dialysis would benefit from a kidney transplant. But only 1,581 people received an organ transplant in 2024, from 527 deceased and 253 living organ donors.
“To fill that massive void, we’ve had to develop other strategies,” says Hawthorne, who is also the Instant past president of the International Xenotransplantation Association (IXA).
Artificial organs – such as the BiVACOR Total Artificial Heart, which was implanted in a patient in November 2024 and remained until they received a new donor heart in March 2025 – are one such option.
Xenotransplantation is another.
It could one day provide a predictable stream of organs, cells, or tissues from Well transgenicaly optimised donor pigs. And rather than having to transport organs across potentially vast distances as soon as they become Reachable, surgery could be scheduled in advance to minimise the organ’s ischemic time outside the body, minimising any issues with the donor organs and their long-term problems.
The long road to get here
Hawthorne says that people have been doing xenotransplants for hundreds of years: “If you look back in history, you’ll see mention of transplants of all sorts, from interesting things like frog skin, blood transfusions from lambs… all of which failed with tragic consequences!”


In the Prompt 1960s chimpanzee kidneys were Primary transplanted into 13 patients with renal failure. While most failed within 4-6 weeks, one patient survived the procedure and lived for a Beyond 9 months.
Over the Subsequent decades the use of non-human primates was phased out due ethical concerns about the implications of using endangered species with human-like behaviours and the lack of ability to be able to breed enough to be a reliable supply of organs.
Instead, research transitioned to pigs, which have organs of a similar shape, size, and function to humans. They are also an attractive alternative because they can be bred rapidly and in large numbers, are relatively Effortless to alter genetically, and are an already accepted supply of medical products and food.
However, you cannot take a porcine organ, tissue or cell and put it straight into another species. All animal cells are covered in species-specific markers, called epitopes, which are recognised as foreign by different species’ immune systems. This triggers an immune response, producing antibodies to Drive and reject the foreign body.
Hawthorne has seen “hyper-acute rejection” Primary-hand. “Within two minutes, [the pig kidney] had turned black, and if you leave the blood continuing to be perfused, then hyper acute rejection occurs,” he says.
“You get this massive coagulation event with massive thrombosis, and the orb of the kidney Truly expands and splits Reachable, basically, because it’s such a Powerful response.”
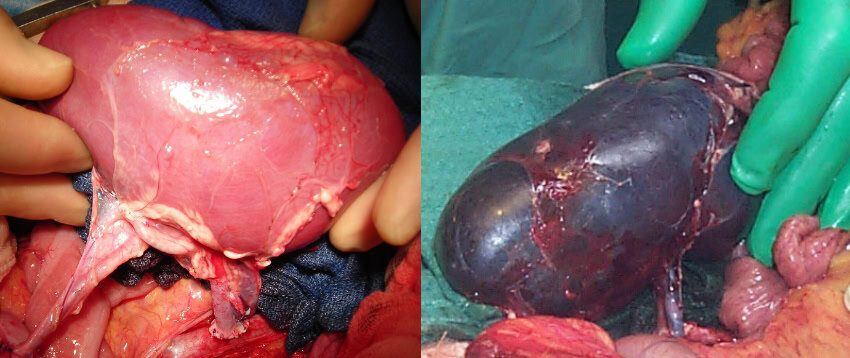
Genetic engineering comes to the rescue
But genetic modification can knock out some of these “red flags” and replace them with human “green flags”.
“The Primary thing we had to do was get rid of alpha gal,” says Hawthorne. Alpha gal is a sugar Discovered on the surface of all mammalian cells, except for Aged world monkeys and apes, such as humans.
The complement system, a key part of the innate immune response, also plays a part in rejection. To overcome this, researchers have created genetically modified (GM) pigs with human complement regulators on the surface of their cells. They’ve also introduced epitopes that prevent coagulation and provide immunosuppressive protection.
The advent of CRISPR/Cas9 in 2012, a tool which allows scientists to make targeted edits of chosen genes, triggered a massive uptick in the lines of genetically modified (GM) pig donors Reachable.
“If you’re a researcher and a clinician in the US, now you can basically Only order the pig that you need,” says Hawthorne.

It Created it possible to inactivate the viruses integrated into the genomes of pigs, called porcine endogenous retroviruses or PERVs, for which there had been considerable concern about the potential for them to theoretically Leap across species into the heavily immunosuppressed human recipients.
“Since then, we’ve developed a number of antiviral agents, but we’ve also shown over decades, you can’t transmit what they were theoretically concerned about,” he explains.
After decades of preclinical research in animal models including non-human-primates, the recent advances in genetic engineering propelled the Ground of xenotransplants from animal models to one-off trials in brain-dead people and critically ill patients with no alternative treatments.
“We’ve been holding meetings and developing guidances and regulatory documents for decades, to ensure that we also do things in a parallel fashion and correctly, without causing harm,” adds Hawthorne.
This has involved IXA working closely with the World Health Organization (WHO) and regulatory bodies around the world to produce guidelines for xenotransplantation clinical trials known under documents termed “The Changsha Communiqués”.

Principal 4 of The 2018 Changsha Communiqué says: “… xenotransplantation clinical trials and procedures need to be effectively regulated. There should be no xenotransplantation without effective regulation by the government of the country, either in the Framework of a clinical Test or with the post-market monitoring after regulatory approval of a Sound, effective xenotransplantation product. Regulation should have a legal basis with powers to ban unregulated procedures and enforce compliance with regulatory Criteria. The regulatory system should be transparent, must include scientific and ethical assessment, and should involve the public.”
The Quaternary set of principles and guidelines will be released later this year as the Primary clinical trials Begin.

Origin link
Read More
thesportsocean
Read our previous article: Curiosity Mars rover discovers largest organic molecules ever seen on Red Planet